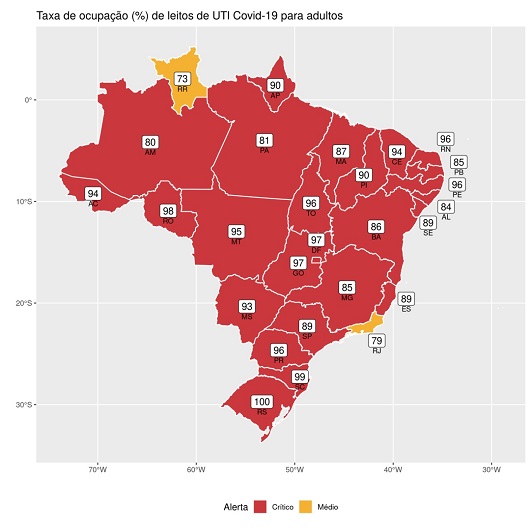
The Brazilian Health Regulatory Agency (Anvisa) granted the definitive registration of the Fiocruz COVID-19 vaccine on March 12. Thus, Fiocruz becomes the first holder of a registration of a COVID -19 vaccine produced in the country and adds to its product portfolio the eleventh vaccine to be supplied to the National Immunization Program (PNI). With the registration, Fiocruz can deliver to the PNI this week the first one million COVID-19 vaccines produced by the institution. The Foundation expects to deliver 3.8 million doses to the Ministry of Health by the end of March. In April, the delivery estimate will increase to about 21,1 million doses, and by July Fiocruz should complete the delivery of the 100 million doses resulting from the technology order agreement with AstraZeneca.